Natural biocide disrupts nestmate recognition in honeybees
Biological control products are becoming more popular, replacing many chemical pesticides which are harmful to pollinators. Microorganisms found in biocontrol products produce secondary metabolites and enzymes that help defend plants against pathogens and pests. One such biocontrol is Beauveria bassiana which is a fungal strain that has been used in organic agriculture since the 1980s. B. bassiana infects the cuticular hydrocarbon layer (CHC) of a pest with spores that kill the host in a matter of days (Figure 1). B. bassiana can be produced for a low cost and is great at targeting specific pests while remaining relatively harmless to non-target organisms and humans. Honeybees are among the non-target organisms that forage on B. bassiana treated crops. Spore contamination on the body of honey bees does not seem to be a threat to bee survival. For this reason, honey bees have been considered ideal candidates to vector B. bassiana to other crops to target pests and even control in-hive Varroa destructor populations.

Image source
Although our understanding B. bassiana is that it is relatively safe for pollinators, it is still important to identify any potential risk these biocontrol products may have on pollinators since minimal exposure to chemical pesticides can negatively impact foraging ability and behavior. Exposure to B. bassiana causes the immune system to alter the CHC and chemical signature in infected individuals thereby disrupting colony recognition. The CHC acts as a marker for insects to discriminate healthy from sick individuals. Eusocial insects such as honey bees can recognize sick colony members and will try to eliminate them from the hive to avoid the spread of pests or pathogens.
Cappa et al. (2019) measured bee guard’s response toward foragers (nestmates and non-nestmates) exposed to B. bassiana. Behavioral assays were used to assess guard bee recognition of nestmate/non-nestmate foragers exposed to B. bassiana. Four stimuli were presented to the hive entrance randomly: two nestmate and two non-nestmate foragers either unexposed or exposed to B. bassiana three days prior. Video recording was used to assess number of agonistic contacts (biting, stinging). In addition to behavioral analysis, chemical analysis was used to identify CHC differences in exposed and unexposed bees.
Chemical analysis determined that exposed bees had altered CHC due to B. bassiana exposure, had fewer instances of agonistic contacts and were accepted more frequently into foreign colonies than unexposed bees. This result demonstrates a disrupting effect of B. bassiana on nestmate recognition. Increased acceptance of chemically unrecognizable foragers exposed to B. bassiana could lead to increased forager drift and spread of pests or pathogens to neighboring colonies. Future studies will aim to investigate the rate at which drifting is occurring in bees exposed to the fungal spores of B. bassiana and other parasitic fungi.
Identification of the first endolysin Cell Binding Domain (CBD) targeting Paenibacillus larvae.
Bacteriophages vastly outnumber bacteria on the planet. Prior to the use of antibiotics, bacteriophages were being used in the medical industry to target and destroy pathogenic bacteria in humans. Due to the rapid evolution of bacteria, antibiotic resistance has become a central problem in the medical industry. Bacteriophages are now receiving much more attention due to their ability to combat deadly pathogens which are no longer threatened by antibiotics. Bacteriophages are host specific viruses that inject their DNA into bacterial cells, reproduce, and produce enzymes called endolysins that break down the cell wall, causing the cell to rupture and the progeny phages to continue the lifecycle again on new host bacterial cells (See video supplement). These endolysins have two functional structures to lock onto host bacteria: an enzymatically active domain (EAD) and a cell wall binding domain (CBD). These domains have a high level of host specificity.
Not only are bacteriophages being used to help stop pathogens in humans, but insects too. Santos et al. (2019) have been testing the ability of bacteriophage endolysins to kill the bacteria responsible for American Foulbrood, Paenibacillus larvae. The only treatment or control option that currently exists for American Foulbrood is incineration of infected hives and equipment. Santos et al. (2019) are searching for an endolysin capable of binding specifically to the P. larvae cell wall. Results from this work have identified an endolysin with a CBD capable of binding to P. larvae. The efficacy of phages against AFB was discussed in an interview with molecular biologist Dr. Sandra Hope, in which she states:
“…the phages could reverse the damage of infection within three days and completely clear up the infection within 10 days, and recurrence was not detected at any point during the 10-month study” (Bee Culture, April 2019 issue, pg. 55).
Continuation of this work will provide new methods to detect, isolate and destroy P. larvae rather than the entire colony. The ability for bacteriophages to bind to AFB spores opens up many opportunities for researchers to develop phage treatments to prevent future AFB outbreaks.
The company Esplin Biotechonology, LLC has plans to release the first bacteriophage on the market to target AFB called “BroodSafe” which is to be sold as a “feed additive” rather than a treatment for AFB. Broodsafe will be a blend of powdered sugar and super concentrated, laser focused AFB-killer bacteriophages. Beekeepers will be able to mix BroodSafe in with their liquid feed or sprinkle it on the top frames similar to probiotic treatments. Three feedings of BroodSafe is recommended in 3-day to 3-week intervals to target multiple brood cycles.
Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure
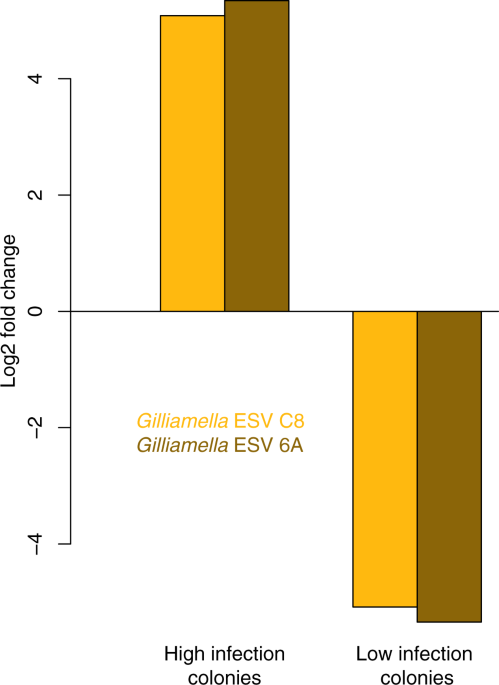
Nosema ceranae is the most common honey bee disease that infects the gut, degenerating digestive tissue and inhibiting tissue renewal. Degradation of the gut leads to malnutrition, increased mortality and decreased colony strength. To investigate the relationship between the honey bee gut microbiome and N. ceranae, Rubanov et al. (2019) infected honey bees with N. ceranae, returned them to the hive and used genetic analysis to determine Nosema levels in bees 5, 10 and 21 days after infection. Results showed variation in colony infection levels with no significant differences in microbiota from colonies with high or low levels of infection. In line with previous research, this study confirmed the relationship between gut dysbiosis and concentration of two variants of Gilliamella (ESVC8, ESV6A), a core gut symbiont (Figure 2). Further research will seek to identify if Gilliamella is a causative agent in Nosema infection and if it can be removed from the microbiome to promote health and resistance to Nosema.